Biomedical Research Cluster
The Biomedical Research Cluster (BRC) at the University of Canberra is an exciting interdisciplinary group for biomedical research. BRC brings together a core team of five highly productive researchers, with scientific expertise in the development of therapeutic solutions for medically significant, complex diseases which addresses current and emerging global challenges in medical research. As a group, BRC members have a long history of collaborative work, with extensive national and international networks, and strive to build breakthrough knowledge in complex chronic inflammatory diseases through harnessing these relationships to enhance interdisciplinary research.
The core Academic members of the BRC are:
Areas of expertise: Molecular virology, nuclear signalling, asthma, drug discovery
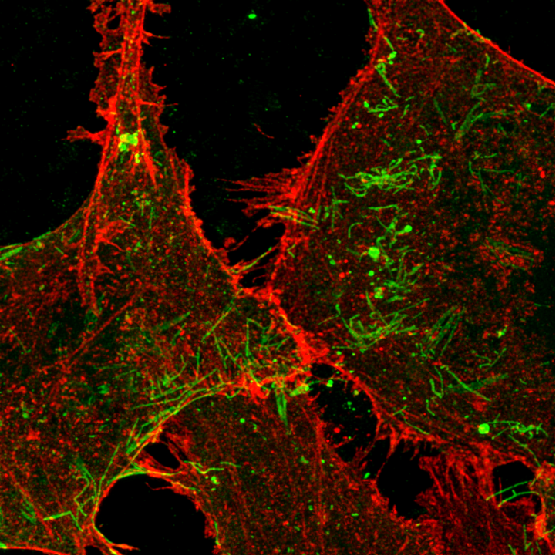
Professor Reena Ghildyal leads the Respiratory Virology Group. The group’s research focus is molecular pathogenesis of respiratory viruses, with special reference to asthma exacerbations. The group focuses on the host-virus interface in rhinovirus and respiratory syncytial virus infections to identify novel and viable drug targets. Recent work by a graduate student has identified a popular, widely used naturally occurring compound as an effective antiviral to treat respiratory syncytial virus disease. Building on work done by previous Honours students and postdoctoral fellows in the group, current pre-clinical work in the mouse model of asthma is focussed on developing an oral treatment for severe asthma.
Reena collaborates with research groups within Australia (Monash University, Telethon Kids Institute) and internationally (University of South Florida, University of Georgia, Imperial College, Fudan University) and her research is funded by the National Health and Medical Research Council, the Australia-China Science Research Fund and the Australia-India Science and Technology Research Fund.
Publication highlights
Croft SN, Walker EJ and Ghildyal R. RIPK1 is cleaved by 3C protease of rhinovirus A and B strains and minor and major groups. Viruses 2021, 13(12):2402. doi: 10.3390/v13122402
Croft SN, Walker EJ and Ghildyal R. Human rhinovirus 3C protease cleaves RIPK1, concurrent with caspase 8 activation. Scientific Reports 2018, 8:1569, doi:10.1038/s41598-018-19839-4
Together, these two papers have led to the elucidation of a key aspect of the host-virus interface in rhinovirus infection, delineating the molecular mechanism that underlies the regulation of apoptosis in rhinovirus infection. Current work in the laboratory is focussed on extending this work to identify viable drug targets. Rhinovirus infections are the major cause of asthma attacks, and our work could stop the virus from causing severe disease.
Walker EJ, Heydet D, Veldre T, Ghildyal R. Transcriptomic changes during TGF-β-mediated differentiation of airway fibroblasts to myofibroblasts. Scientific Reports 2019, Dec 30;9(1):20377. doi: 10.1038/s41598-019-56955-1
Breton JD, Heydet D, Starrs LM, Veldre T, Ghildyal R. Molecular changes during TGFβ-mediated lung fibroblast-myofibroblast differentiation: implication for glucocorticoid resistance. Physiological Reports 2018, 6(7):e13669.
doi: 10.14814/phy2.13669
These two papers together identify novel molecular pathways that underlie steroid insensitive asthma. 1 in 9 Australians suffer from asthma. Despite many years of development, there is a subgroup of asthma sufferers who do not respond to any of the current treatments and whose asthma cannot be controlled. Based on our data, we are currently in pre-clinical phase to collect data for development of a new asthma treatment.
Research engagement and impact
Experiments by Reena Ghildyal and her team have shown that lung fibrosis, characteristic of asthma, can be reversed by targeting a key intermediate in the fibrosis pathway. The group has identified an experimental anti-cancer drug that specifically targets the key intermediate, presenting an opportunity for drug re-purposing. The group is currently running proof of principle tests in an animal model of asthma funded by ANU Connect Ventures.
Areas of expertise: Vision neuroscience, epigenetics, myopia, therapeutic translation

Associate Professor Regan Ashby heads the Visual Neuroscience Group. This group, including Dr Cindy Karouta and Dr Kate Thomson, investigates the mechanisms underlying the development of the visual disorder myopia (short-sightedness). Using a suite of molecular, biochemical, analytical, and physiological techniques, Dr Ashby’s group studies the environmental triggers and biological processes that bring about this disorder, with a view towards developing novel and more effective interventions to meaningfully enhance patient outcomes. The group is best known for their seminal work demonstrating that bright light exposure, experienced by being outdoors, protects against the onset and development of myopia. From this work, Dr Ashby and his team have developed several novel ophthalmic treatments for this visual disorder, the most advanced of which are currently undergoing clinical testing in patient populations.
Publication highlights
Ashby RS, Ohlendorf A and Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative Ophthalmology and Vision Science 2009, 50(11):5348–54. doi: 10.1167/iovs.09-3419
This is the seminal work showing that bright light exposure inhibits myopia development by stimulating the release of the neurotransmitter dopamine within the eye. This is one of the most cited papers on myopia in 2009 and is recognised by ISI as a Highly Cited Publication. The impact of this work has been acknowledged by several prestigious awards, including the Attempto prize for Neuroscience (Germany) and the Carl-Zeiss Investigators Award in Vision Science (USA)).
Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, Rose KA. The epidemics of myopia: Aetiology and prevention. Progress in Retinal and Eye Research 2018, 62:134–49. doi: 10.1016/j.preteyeres.2017.09.004
This paper reviews the aetiology of myopia and suggests evidence-based approaches to prevention, based on increased time outdoors in primary schools, with longer-term reform of school curriculum and systems to reduce early educational pressure. This is the most highly cited paper on myopia published in 2018 and is recognised by ISI as a Highly Cited Publication.
Thomson K, Karouta C, Morgan I, Kelly T, Ashby R. Effectiveness and safety of topical levodopa in a chick model of myopia. Scientific Reports 2019, 9(1):18345. doi: 10.1038/s41598-019-54789-5
Based on the finding that bright light exposure can inhibit myopia, Regan and his team have developed a novel pharmacological intervention. This paper details the preclinical data regarding the safety and efficacy of this ophthalmic treatment and forms the basis of a granted US patent (10,874,629 B2). This pharmacological intervention is currently entering Phase II trials after successful completion of a Phase I safety and tolerability trial.
Research engagement and impact
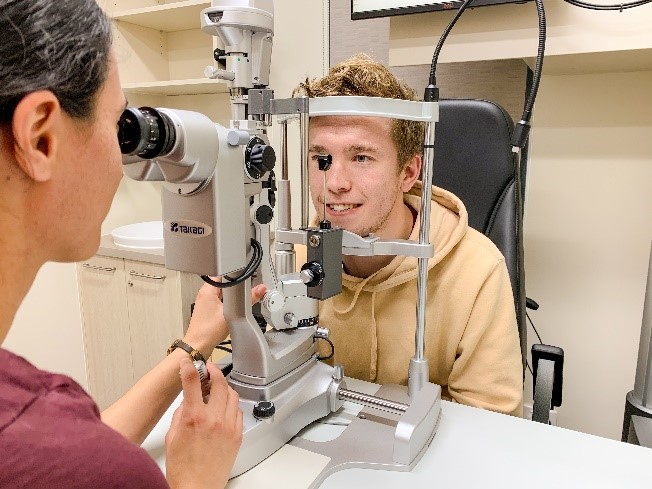
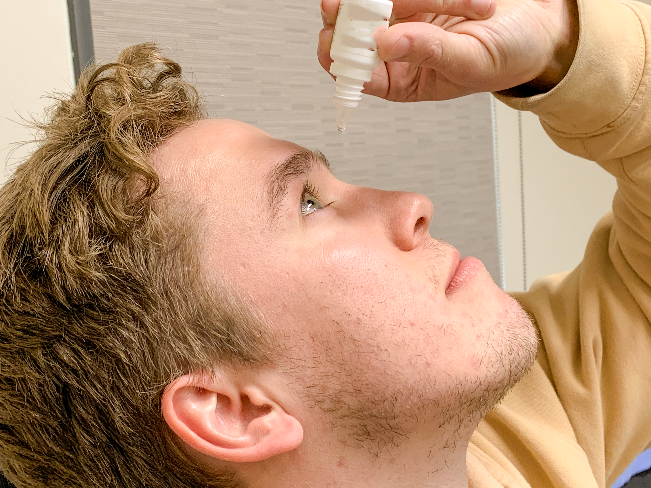
Development of a new ophthalmic treatment for myopia by Regan Ashby, Cindy Karouta and Kate Thomson. Their basic research discovery, that time spent outdoors delays development and retards progression of myopia, led them to investigate pharmacological strategies to mimic this effect. They subsequently showed that their lead compound mimics the effect of sunlight on myopia in animal models, resulting in a multinational patent (granted in USA, Malaysia, Japan, South Korea). Following on from a successful Phase I Clinical Trial through the University of Canberra Hospital (UCH), the group is working with international consortia towards Phase II/III clinical trials.
As a member of a large international research consortium (International Myopia Institute), Regan contributed to International Myopia Institute White Papers which guide the WHO redevelopment of guidelines for the treatment of myopia). Regan was invited to participate in the Johnson & Johnson Vision Research Program Myopia Summit in 2022. Regan Ashby and Matt Rutar are members of the newly formed Vision ACTion consortium whose focus is vision research in the ACT region, with a view to enabling development of novel treatments and diagnostics.
Areas of expertise: Molecular virology, animal models, chronic liver disease, hepatocellular carcinoma
Associate Professor Xiaonan Zhang heads the Hepatitis B Virus Group within BRC. His group focuses on clinic-oriented molecular biology and translational medicine of the hepatitis B virus (HBV).
Xiaonan’s research focus is on chronic infection by HBV, which results in hepatic cancer,
a major medical problem in Australian Indigenous populations. His pre-clinical work in a novel animal model is aimed at developing a cure for chronic HBV and his long-term focus on HBV has yielded unique insights that changed some key perceptions on the virus, one of which led to the discovery of a novel viral particle (Capsid-Antibody-Complexes, CACs) that has a substantial role in HBV pathogenesis. In addition, he also made significant contribution in emerging viral infections such as enterovirus 71 (hand foot and mouth disease), H7N9 Influenza virus and COVID-19.
Publication highlights
Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, Jia X, Wu M, Shi B, Xu S, Chen J, Wang W, Chen B, Jiang L, Yu S, Lu J, Wang J, Xu M, Yuan Z, Zhang Q, Zhang X, Zhao G, Wang S, Chen S, Lu H. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020, 583:437–40. doi: 10.1038/s41586-020-2355-0
Xiaonan Zhang led a team consisting of clinicians, virologists, bioinformaticians, and genome biologists from six institutions in Shanghai in response to the initial spread of SARS-CoV-2 from Wuhan to Shanghai (January–March 2020). As the leading author, Xiaonan coordinated the taskforce in processing the clinical samples and key information, liaised with key expertise in data analysis, and drafted the manuscript in just 50 days from the first sample to initial submission.
Zhang X, Lu W, Zheng Y, Wang W, Bai L, Chen L, Feng Y, Zhang Z, Yuan Z. In situ analysis of intrahepatic virological events in chronic hepatitis B virus infection. Journal of Clinical Investment 2016, 126(3):1079–92. doi: 10.1172/JCI83339
Xiaonan Zhang devised a novel in situ hybridisation assay which enabled the visualisation of the HBV nucleic acids, including cccDNA within the histological context. This study highlighted the cellular heterogeneity within the hepatocytes and suggested its mechanistic association with long-term persistence of HBV in vivo. It was selected as a feature article in the issue of JCI in which it was published, with an accompanying commentary by Prof. Peter Revill and Prof. Stephen Locarnini from Doherty Institute. Several invited academic talks were given based on this work (for example, the 2015 HBV International Meeting and the 2017 Asian Pacific Association for the Study of the Liver [APASL] Conference).
Research engagement and impact
By marrying the cutting-edge mRNA vaccine technology with his unique small animal model of HBV Xiaonan Zhang and his team are actively developing a next generation therapeutic vaccine to cure chronic hepatitis B. This strategy will potentially bring a paradigm shift in the advancement of HBV-cure initiative.
Areas of expertise: Microbiome, bacteriology, Chron’s disease, inflammatory bowel disease

Associate Professor Claire O’Brien is Group Leader of the Host-Microbe Interactions group within BRC. Her research focus is the gut microbiome and the molecular basis of inflammatory bowel disease (IBD), a chronic condition, and aims to identify the nature of gut microbiome imbalance in different disease states. Her research attempts to determine whether observed changes cause disease or are a consequence of the disease process, as well as understand ways in which the gut microbiome can be restored following perturbation.
Claire’s research is aimed at developing novel strategies to restore gut microbiome balance and she uses in vitro co-culture of bacteria and macrophages, combined with molecular genetic techniques, to increase the mechanistic understanding of host-bacteria interactions in these diseases.
Publication highlights
O’Brien CL, Bringer MA, Holt KE, Gordon DM, Dubois AL, Barnich N, Darfeuille-Michaud A, Pavli P. Comparative genomics of Crohn's disease-associated adherent-invasive Escherichia coli. Gut 2016, Aug;66(8):1382–9. doi: 10.1136/gutjnl-2015-311059
For this study, Claire used whole bacterial genome sequencing to show that a group of E. coli that is more frequently isolated from patients with Crohn’s disease, a major form of inflammatory bowel disease, did not harbour any genetic element that dictates their ability to survive and replicate within immune cells. Claire also changed the way we think about E. coli associated with disease, suggesting that in diseases where there is ulceration, the ability to survive and replicate within immune cells is potentially more important than the ability to adhere and invade intestinal epithelial cells.
O’Brien CL, Allison GE, Grimpen F, Pavli P. Impact of colonoscopy bowel preparation on gut microbiota. PLoS One 2013, May 1; 8(5):e62815. doi: 10.1371/journal.pone.0062815
This study investigated the impact of colonoscopy bowel preparation on the gut microbiome in patients with and without inflammatory bowel disease. Claire showed that the microbiome does revert to its pre-colonoscopy state in most subjects, however those with inflammatory bowel disease may take longer to, or not fully, recover. This is an important finding, as most individuals over the age of 55 will undergo a colonoscopy at some point in their life, and gut microbiome balance is important for health.This article was identified by PLoS One as a ‘PloS One most cited article’.
O’Brien CL, Pavli P, Gordon DM, Allison GE. Detection of bacterial DNA in lymph nodes of Crohn’s disease patients using high-throughput sequencing. Gut 2014, Oct;63(10):1596-606. doi: 10.1136/gutjnl-2013-305320
This was the first study of the lymph node microbiome in health and disease. Claire showed that a large fraction of patients with and without inflammatory bowel disease, who have undergone surgical resection of their bowel, have a detectable microbiome in their lymph nodes. It was previously thought that lymph nodes were sterile, so this challenged the thinking of the researchers and led to other studies by Claire’s group, including a study looking at viable bacteria in lymph nodes (Kiely et al. 2018). As part of this study, Claire also cultured bacteria from lymph nodes of IBD patients, but not controls, and one of Claire’s PhD students is now comparing the genomes of these isolates with bacteria isolated from healthy controls.
Research engagement and impact
Research conducted by Claire O’Brien and her team laboratory utilising inflammatory bowel disease (IBD) patient specimens has identified the expression of a number of family-specific alleles that could be likely drivers of disease. For some families, it is possible that blood transfusions and already developed therapies may be of benefit. Claire is a member of the Australian IBD Microbiome (AIM) Consortium that is recruiting participants to conduct the largest national study ever on the topic with a view to develop novel treatments; the clinical protocols have been published in BMJ (2021).
Areas of expertise: Neuroimmunology, retinal degenerative diseases, age-related macular degeneration, ocular therapeutics and gene therapy

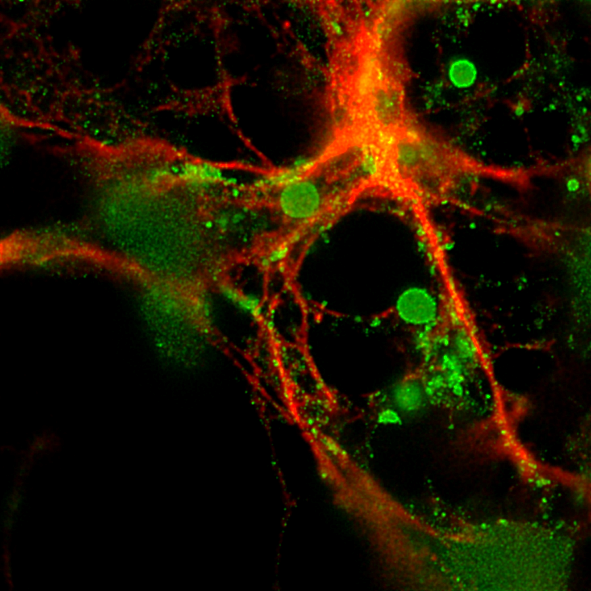
Assistant Professor Matt Rutar leads the Retinal Disease Laboratory, which seeks to understand the molecular and cellular factors that underscore vision-threatening disorders of the eye. Presently, this work focuses on the immunological landscape of the eye, and the environmental and neurodegenerative indicators that orchestrate inflammation in the retina. Chronic inflammatory processes have recently emerged as an important factor underlying the pathogenesis of many retinal diseases, such as age-related macular degeneration (AMD). AMD is a leading cause of blindness worldwide and accounts for around 50% of all cases of irreversible vision loss in Australia and has few available treatments. Using innovative molecular and cellular biology approaches, Matt’s laboratory aims to uncover new processes of immune regulation in the retina, while simultaneously harnessing this knowledge for more effective therapies for retinopathies including AMD.
Publication highlights
Rutar, M., Natoli, R. & Provis, J.M. Small interfering RNA-mediated suppression of Ccl2 in Müller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J Neuroinflammation 9, 221 (2012). https://doi.org/10.1186/1742-2094-9-221
This work showcased a novel role of Muller cells, a glial cell of the retina, in trafficking of immune cells via chemokine ligand gradients. Using targeted gene therapy, the work showed that inhibition of chemokine gradients suppressed inflammation and cell death in preclinical retinal disease models. The research was influential in establishing a new methodology for facilitating gene therapy in the eye, earning a competitive travel grant and invited platform presentation at the ARVO international meeting (USA, 10,000 researchers and clinicians in attendance).
Karlstetter, M., Scholz, R., Rutar, M., Wong, W.T., Provis J., Langmann, T. Retinal microglia: Just bystander or target for therapy? (2015) Progress in Retinal and Eye Research, 45, 30-5 https://doi.org/10.1016/j.preteyeres.2014.11.004.
This paper reviewed the contrasting roles of retinal immune cells, known as microglia, in retinal health and disease, and their emerging potential a target for therapeutic intervention. This work is widely influential, accruing >300 citations with a field-weighted citation index of 10 (Scopus).
Natoli, R., Fernando, N., Madigan, M., Chu-Tan, J., Valter, K., Provis, J., Rutar, M. Microglia-derived IL-1β promotes chemokine expression by Müller cells and RPE in focal retinal degeneration. Mol Neurodegeneration (2017) 12, 31. https://doi.org/10.1186/s13024-017-0175-y.
This study, published in Molecular Neurodegeneration (Impact Factor 18.9), was first to identify an instrumental role of retinal microglial cells in promoting inflammation in AMD. Il-1b is a pro-inflammatory cytokine that is strongly linked to the pathogenesis of AMD, though the cellular mediators responsible for its activation in the eye were unclear. This work was highly impactful, earning an invited symposia at the ARVO annual meeting in the USA, and is highly cited with a field-weighted citation index of 3.1 (Scopus).
Research engagement and impact
The ocular immunity is a unique and complex system that, despite the pivotal role it plays in retinal diseases, remains largely unexplored. Matt’s research has revealed a rich network of ocular immune cells responsible for orchestrating inflammatory responses that promote retinal cell death and visual impairment. This work initiated a paradigm shift by establishing new biomarkers and therapeutics targets for retinal diseases such as AMD, which remains a key area of focus in the field today. Matt’s laboratory is presently investigating innovative therapeutic approaches for screening and inhibiting these inflammatory mediators in AMD, including targeted gene therapy approaches. This promising work is currently undergoing preclinical evaluation in collaboration with a web of clinical and basic research collaborations, including the newly formed Vision ACTion consortium.