Marcus Butler
10 August 2017: Studying the inner workings of one of Australia’s most important pest-control tools has helped Canberra researchers develop new understandings of how the rabbit calicivirus works.
Rabbit haemorrhagic disease virus was first used in Australia to control feral rabbit numbers in the mid-1990s and a new strain was released earlier this year to boost control measures.
The virus kills more than 95 per cent of infected adult rabbits by attacking the liver and is typically fatal within three days.
Assistant Professor of Immunology Michael Frese said the virus had been under the microscope for some time and the discoveries made recently are changing peoples’ understanding of how it works.
“The work which I’ve been doing, along with University of Canberra PhD candidate Nadezda Urakova and Tanja Strive and Andrew Warden from CSIRO, examined how the virus behaves inside animal cells and why it is so deadly,” Dr Frese said.
“We found that the virus interacts with a particular organelle inside cells, called the Golgi, which is a protein-processing factory. The Golgi compartment is separated by the rest of the cell’s interior by membranes that normally form a neat stack in one part of the cell.
In cells infected with the rabbit calicivirus, the research revealed that the Golgi is blown apart and its remnants are found distributed throughout the cell.
“The Golgi processes many proteins which are later discharged from the cell,” Dr Frese said.
“Virus-infected cells usually produce proteins that serve as early warning signals for neighbouring cells that a viral infection is present.
“Raising the alarm helps other, not yet infected cells to install antiviral defences. So interfering with that process would make a virus more successful at infecting more cells.”
After discovering that rabbit caliciviruses destroy the Golgi, Dr Frese and his collaborators continued their investigations to uncover which part of the virus is responsible for this. They uncovered an entirely new mechanism in the process.
“Through a series of tests we found the virus’ own polymerase, which is known for its function in copying the virus genome, appears to target Golgi membranes, but our findings left us with a problem,” Dr Frese said.
“The particular part of the polymerase that is supposed to interact with membranes is normally hidden inside the polymerase.”
At this stage, the interdisciplinary team turned to advanced computer modelling and came up with an hypothesis that can explain how an internal part of the polymerase interacts with membranes.
“At first, positively charged amino acids on the outside of the polymerase attract and correctly position the virus protein to membranes,” Dr Frese said.
“Next, as series of molecular movements expose a hidden interaction motif which is then free to bind to membranes. It was a surprise to find this kind of complex mechanics at play in the polymerase, but given that the virus has only a limited number of parts, it’s an elegant solution.”
Rabbit caliciviruses are important in Australia where pest rabbits are estimated to cost the economy about $200 million a year.
Other nations including China, where rabbits are farmed for fur and meat, and Spain and Portugal, where they are a native species and an important part of the food chain, are also interested in calicivirus research.
Dr Frese said one of the issues the researchers had to deal with in studying rabbit calicivirus is that it does not grow in cell cultures.
“It’s a tricky virus. It can tell the difference between cells in an animal and cultured cells; it just doesn’t infect lab-grown cells,” he said.
“We hope that by improving our general understanding of the virus we may be able to develop a way to overcome this aversion to cell cultures, which will make future research much simpler.”
The team’s discoveries have been published in a series of academic articles in scientific journals including Virology, PLoS One and Viruses.
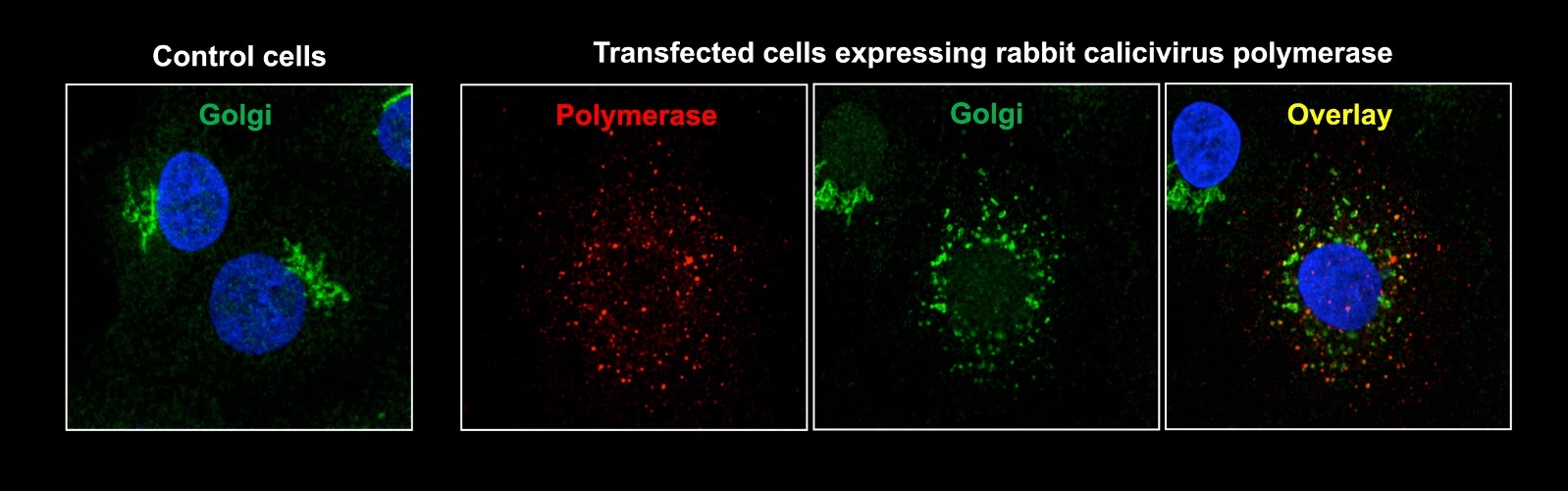
IMAGE ONE (above): Cultured cells with and without the rabbit calicivirus polymerase were stained for Golgi membranes (green), the virus polymerase (red) and DNA (blue). In untransfected control cells a normal stack of Golgi membranes can be seen to the side of the nucleus (stained blue because it contains almost all of the cell’s DNA). Transfected cells that express sufficient amount of polymerase do no longer have a normal Golgi, they contain a large number of dispersed Golgi vesicles instead of a neat stack of Golgi membranes.
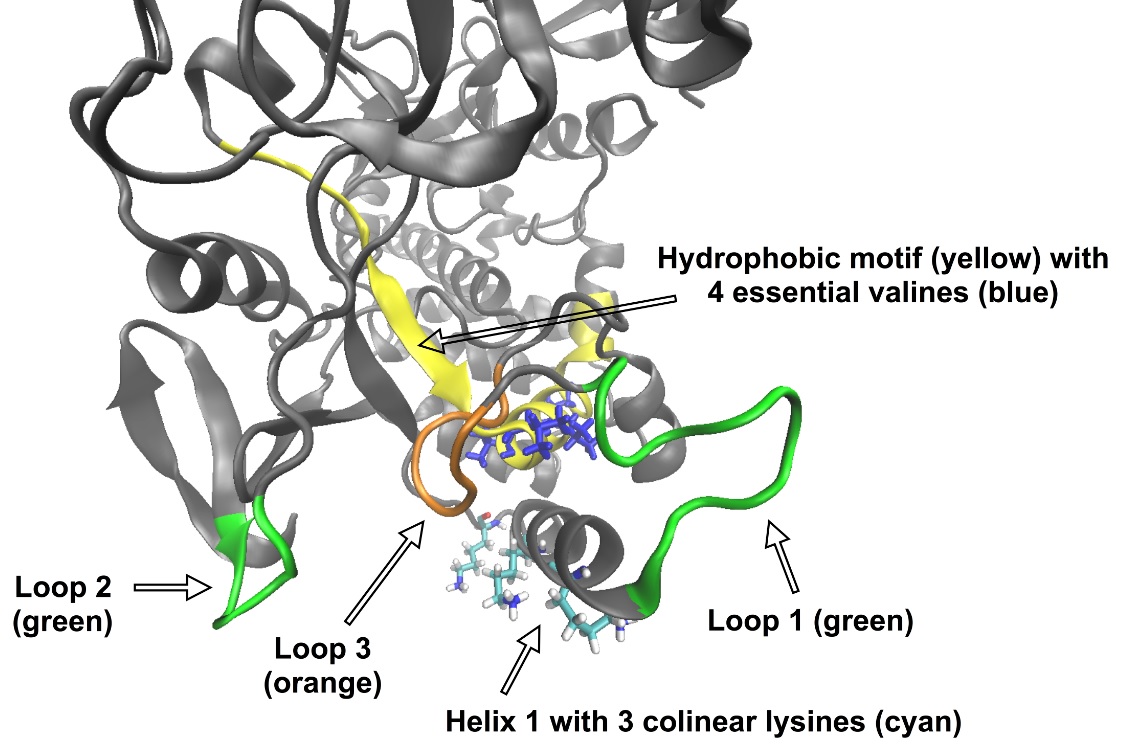
IMAGE TWO (above): Ribbon diagram of the rabbit calicivirus polymerase. The polymerase part (‘hydrophobic motif’, coloured in yellow) that is supposed to interact with Golgi membranes normally resides inside the polymerase protein, but new computer-aided modelling revealed that other parts of the polymerase (‘loop 1’ and ‘loop 2’, green) can move out of the way, first exposing the hydrophobic ‘loop 3’ (orange) and then the previously hidden hydrophobic interaction motif.


